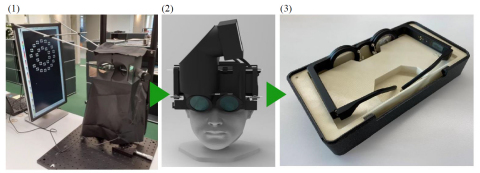
(1) Kubota Vision conducted a clinical study in early 2020 with an electronic tabletop optical projection device that embodied Kubota Glasses technology; (2) The company also completed a successful proof-of-concept (POC) clinical study to validate the concept of a wearable myopia-control device based on Kubota Glasses technology in August 2020; (3) Based on these results, the company has completed the first spectacle-style wearable prototype based on Kubota Glasses technology. (Photo: Business Wire)

SEATTLE–(BUSINESS WIRE)–Kubota Vision Inc. (Kubota Vision), a clinical-stage specialty ophthalmology company and wholly-owned subsidiary of Kubota Pharmaceutical Holdings Co., Ltd. (Tokyo 4596), today announced that the company achieved a milestone by completing a first spectacle-style wearable prototype based on Kubota Glasses technology.
Kubota Vision conducted a clinical study in early 2020 with an electronic tabletop optical projection device that embodied Kubota Glasses technology (Photo 1). The results of the study demonstrated that axial length decreases with the application of projected myopically-defocused images in the test eye compared to the control eye, which has not been reported in the literature. The company also completed a successful proof-of-concept (POC) clinical study to validate the concept of a wearable myopia-control device based on Kubota Glasses technology in August 2020 (Photo 2). Based on these results, the company has completed the first spectacle-style wearable prototype based on Kubota Glasses technology (Photo 3).
Kubota Vision continues to advance its program. Further clinical studies will be conducted to verify the changes in axial length induced by myopically-defocused virtual image projection over a longer period of time. The company is also working on product design improvements and plans additional clinical studies for regulatory approvals.
Ryo Kubota, MD, PhD, Chairman, President and CEO of Kubota Vision Inc., stated, “It is always a challenge to replicate the results and performance of a larger device in a smaller device. However, with our experts around the world collaborating and applying the experience, knowledge and skills gained through our PBOS (miniature OCT) device being developed with the National Aeronautics and Space Administration (NASA), we have been successful in advancing the value in this myopia control device as well. Our commitment to treat patients around the world drives our continued efforts, and it is our goal to bring our products to them as quickly as possible.”
Of people aged 20 and under, 96% of South Koreans, 95% of Japanese, 87% of Hong Kongers, 85% of Taiwanese and 82% of Singaporeans are affected by the condition, according to Kubota.
To assess the device’s effectiveness, Kubota is conducting clinical tests on about 25 people in the U.S. “We intend to sell it first in Asia, which has a high ratio of nearsighted people,” said Ryo Kubota, the company’s president.
The Tokyo-based company plans to begin selling the device in Asian markets, including Taiwan, Singapore, Hong Kong, Thailand and Malaysia, in the second half of the year. It is considering whether to offer it through local sales agents or online.
Kubota began clinical trials on the device last July after confirming the therapeutic effect of the mechanism using a desktop system. It is also developing a contact lens-type myopia correction device.
About Kubota Glasses Technology
Kubota Glasses technology works to reduce the increase in axial length associated with myopia by projecting myopically-defocused virtual images generated using micro-LEDS on the peripheral visual field to actively stimulate the retina. Passive stimulation using myopic defocus is already in use in the US with a contact lens, “MiSight® 1 day” by CooperVision, which is U.S. Food and Drug Administration (FDA) approved to slow the progression of myopia. This product, which uses multifocal contact lens technology, passively stimulates the entire peripheral retina with light myopically defocused by the non-central power of the contact lens. Kubota Glasses technology leverages nanotechnology in its electronic glasses-based device and seeks to reduce the progression of myopia by actively stimulating the retina for shorter periods while maintaining high-quality central vision and not affecting daily activities.
About Myopia
Myopia, or nearsightedness, is a refractive vision disorder which causes blurred sight at a distance. It occurs when the length of the eye (known as axial length) is too great; myopia progresses as axial length increases with age, until the early 20s. Myopia currently affects 2.56 billion people worldwide and is projected to affect 3.4 billion people by 2030, if current trends remain unchanged.*1 Myopia increases the risk of developing sight-threatening diseases such as myopic maculopathy, retinal detachment, and glaucoma – making a measurable impact on society.*2 Today children in East Asia, including Japan, China, Hong Kong, Taiwan, South Korea, Singapore, develop myopia at a high rate; for example, 96.5% of 19-year-old males suffer from myopia in Seoul.*3 Myopia also affects over 40% of individuals over the age of 12 years in the U.S.*4
*1 Report of the Joint World Health Organization–Brien Holden Vision Institute, University of New South Wales, Sydney, Australia. The impact of myopia and high myopia. 16–18 March 2015. https://www.who.int/blindness/causes/MyopiaReportforWeb.pdf.
*2 Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012 Nov;31(6):622-60
*3 Dolin E. The myopia boom. Nature 2015 Mar 19;519(7543):276-8
*4 Prevalence. International Myopia Institute. https://www.myopiainstitute.org/prevalence.html. Accessed May 15, 2020.
About Kubota Vision Inc.
Kubota Vision Inc. is a wholly-owned subsidiary of Kubota Pharmaceutical Holdings Co., Ltd. (Tokyo 4596) committed to translating innovation into a diverse portfolio of drugs and devices to preserve and restore vision for millions of people worldwide. Kubota Pharmaceutical group’s development pipeline includes drug candidates for the treatment of diabetic retinopathy, Stargardt disease, and optogenetics-based gene therapy for the treatment of retinitis pigmentosa. The company is also developing a handheld OCT device for the monitoring of neovascular retinal diseases, to be used directly by patients, and wearable device for myopia control. https://www.kubotavision.com/; https://www.kubotaholdings.co.jp/en/
Cautionary Statements
Certain statements contained in this press release are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 and the Private Securities Litigation Reform Act of 1995. Any statements contained in this press release that are not statements of historical fact may be deemed to be forward-looking statements. These forward-looking statements include statements regarding our expectations related to our development plans and ability to successfully develop and commercialize our product candidates and the potential efficacy, future development plans and commercial potential of our product candidates. These statements are based on current assumptions that involve risks, uncertainties and other factors that could cause the actual results, events or developments to differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties, many of which are beyond our control, include, but are not limited to: our investigational product candidates may not demonstrate the expected safety and efficacy; our pre-clinical development efforts may not yield additional product candidates; any of our or our collaborators’ product candidates may fail in development, may not receive required regulatory approvals, or may be delayed to a point where they are not commercially viable; our clinical trials could be delayed; new developments in the intensely competitive ophthalmic pharmaceutical market may require changes in our clinical trial plans or limit the potential benefits of our investigational product candidates; the impact of expanded product development and clinical activities on operating expenses; adverse conditions in the general domestic and global economic markets; as well as the other risks identified in our filings with the Securities and Exchange Commission. These forward-looking statements speak only as of the date hereof and we assume no obligation to update these forward-looking statements, and readers are cautioned not to place undue reliance on such forward-looking statements. For a detailed discussion of the foregoing risks and other risk factors, please refer to our filings with the Securities and Exchange Commission, which are available on Kubota Pharmaceutical Holdings (Kubota Vision’s parent company) investor relations website (https://www.kubotaholdings.co.jp/en/ir/) and on the SEC’s website (http://www.sec.gov).
“Kubota Vision”, the Kubota Vision logo and “Kubota” are registered trademarks or trademarks of Kubota Vision Inc. or Kubota Pharmaceutical Holdings in various jurisdictions.

Media and Investor Relations Contact:
Hiroki Maekawa
Chief Financial Officer
Phone: +81-3-6550-8928
Email: pr@kubotaholdings.co.jp
kubota glasses price: Japanese company launches “smart glasses”, promise every day of 1-hour testing can reduce access. This smart lens was developed by the Japanese company Kubota Pharmaceutical Holdings, effectively improving eyesight by eliminating the underlying cause of myopia.

Ryo Kubota, an expert and CEO of ophthalmology – who develops smart glasses, has been studying the treatment of myopia for a long time.
The product principle is a way of projecting an image from the lens of this part to the wearer’s retina to correct the refractive error causing myopia. According to the company, if you wear the device for 60 to 90 minutes a day, you will improve myopia.
Through further clinical trials, they are trying to determine how long the effects will last after wearing them and how many days users have to wear the device to be able to permanently eliminate nearsightedness.
The biggest advantage of smart glasses is that you can restore your vision spontaneously without surgery.
President Ryo Kubota said he plans to start selling in the Asian market in the second half of this year and affirms it will especially help Asians who are very susceptible to myopia.
The current price has not been determined exactly, but Ryo Kubota said the minimum price is 100,000 円 (about 955.70 USD).
On the other hand, Kubota Pharmaceutical Holdings is developing a contact lens-style myopia correction device with similar effect behind smart glasses.
Currently, the company is considering whether to make the product available through local and online resellers.